Stars and Nebulae...
Index
Atoms These pages explain how astronomers find out what stars and interstellar dust clouds are made of.
Michael Gallagher |
Atoms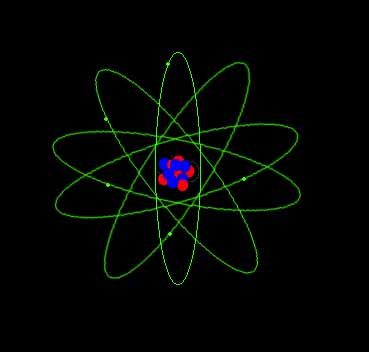
An atom has a central nucleus surrounded by a cloud of orbiting electrons. The nucleus contains positive protons and uncharged neutrons. Electrons are negatively charged. Atoms usually have no charge because each positive proton is balanced by a negative electron. The type or name of an atom is determined by its number of protons: hydrogen has one proton. Oxygen has 8 and Uranium has 92. Every type of atom has a unique number of protons. |
